This year’s summer COVID wave is big; FDA may green-light COVID shots early

With the country experiencing a relatively large summer wave of COVID-19, the Food and Drug Administration is considering signing off on this year’s strain-matched COVID-19 vaccines as soon as this week, according to a report by CNN that cited unnamed officials familiar with the matter.
Last year, the FDA gave the green light for the 2023–2024 COVID shots on September 11, close to the peak of SARS-CoV-2 transmission in that year’s summer wave. This year, the summer wave began earlier and, by some metrics, is peaking at much higher levels than in previous years.
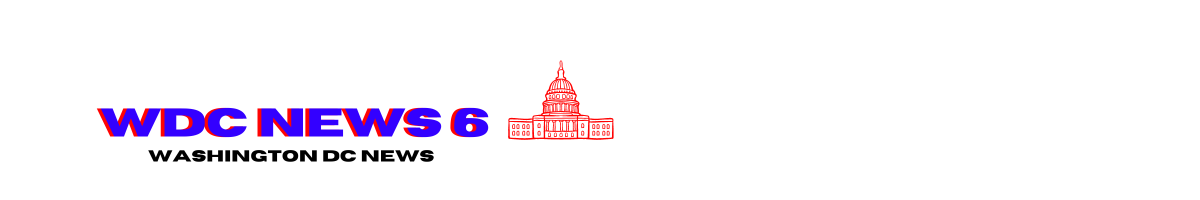
Currently, wastewater detection of SARS-CoV-2 shows “very high” virus levels in 32 states and the District of Colombia. An additional 11 states are listed as having “high” levels. Looking at trends, the southern and western regions of the country are currently reporting SARS-CoV-2 levels in wastewater that rival the 2022–2023 and 2023–2024 winter waves, which both peaked at the very end of December.
Wastewater SARS-CoV-2 levels, by state
Trends in wastewater SARS-CoV-2 levels by region over the last year
Trends in wastewater SARS-CoV-2 levels by region for the entire pandemic
Test positivity—a metric that has weakened given the dramatic decline in testing—shows a weekly test positivity rate of 18.1 percent for mid-August (amid a test volume of roughly 43,000). Such a rate, if truly reflective of cases, has not been seen since the initial towering omicron wave of January 2022, which peaked at 30.5 percent (with a test volume of roughly 991,000).
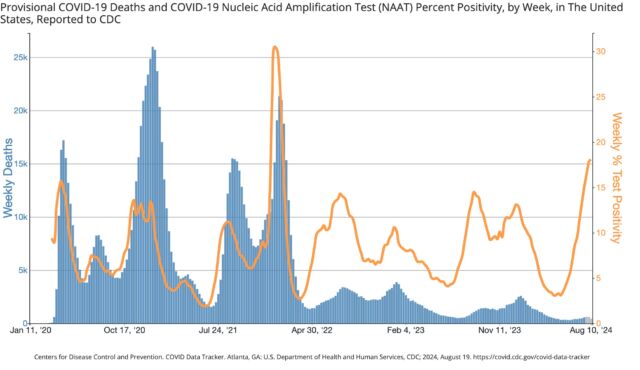
The good news is that given the substantial accumulation of protection from past infections and vaccinations, the two most serious metrics—emergency department visits and deaths—have not shown similar rises. The weekly percentage of emergency department visits with a COVID-19 diagnosis is low and similar to last year’s summer wave. Deaths are likewise low, though they are still only provisional counts for the most recent weeks.
The FDA has firmly embraced a strategy to offer annual COVID-19 vaccines in the run-up to winter waves, not summer waves. The agency’s thinking has always been to encourage Americans to get their flu and COVID-19 vaccines together between September and November, just before a mob of cold-weather respiratory illnesses strike together. The fresh vaccination boost can dull the levels of severe respiratory disease at a time when health care systems are most at risk of becoming overwhelmed.
Seasonality
But, while seasonal flu and some other respiratory viruses reliably surge almost exclusively in the winter, the seasonality of COVID-19 was never a given. And, so far, summer waves have arisen as consistently as winter ones, creating some awkwardness for the vaccine releases.
Some experts have recommended getting a COVID-19 vaccine to protect against the summer surge. “Now is the time to get a dose with this surge,” Michael Osterholm, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, told CNN on Sunday.
However, the only vaccines currently available target last year’s strains (related to the XBB.1.5 omicron variant), which are long gone and may not offer strong protection against current strains (JN.1 and KP.2 omicron variants). Even if the 2024–2025 KP.2-targeting vaccine is approved by the FDA this week and hits pharmacy shelves next week, a dose takes two weeks to produce full protection. By that time, the summer wave will likely be declining. In fact, it looks to have already peaked in some parts of the country, including in some southern and western areas.
The other thing to consider is timing for maximum protection for the likely winter wave. For healthy people five years old and above, the CDC recommended getting only one shot last year. The shots offer peak protection for around four months. If you get your annual shot at the beginning of September, your protection may be on the decline if COVID-19 peaks again at the turn of the year, as it has the past two years.
According to the 2023–2024 guidance, people who are 65 and older can get a second COVID-19 booster four months after getting their first. People who are moderately or severely immunocompromised may also get additional doses of the updated COVID-19 vaccine.
Source link